Government Bans High-Dose Nimesulide Oral Medicines Citing Health Risks
India bans high-dose Nimesulide oral medicines above 100 mg citing health risks. The move aims to improve drug safety and protect public health.
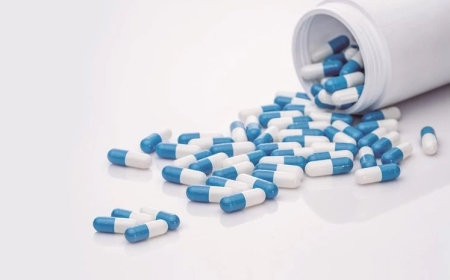
The central government has imposed an immediate ban on the manufacture, sale, and distribution of all oral pain and fever medicines containing Nimesulide above 100 mg in immediate-release form.
The prohibition has been enforced under Section 26A of the Drugs and Cosmetics Act, 1940, following consultations with the Drugs Technical Advisory Board (DTAB). According to a notification issued by the Ministry of Health, the use of high-dose Nimesulide formulations poses a potential risk to human health, especially when safer therapeutic alternatives are readily available.
Nimesulide, a non-steroidal anti-inflammatory drug (NSAID), has faced international scrutiny over concerns related to liver toxicity and other adverse reactions. The government’s decision reflects a broader effort to strengthen drug safety standards and eliminate medicines considered high-risk.
The ban applies only to oral formulations exceeding 100 mg for human use. Lower-dose versions and other approved pain-relief medicines will continue to be available in the market.
Pharmaceutical manufacturers selling high-dose Nimesulide brands have been instructed to stop production immediately and recall affected batches. Industry analysts expect the overall financial impact on major drug manufacturers to be minimal, as Nimesulide contributes only a small portion of total NSAID sales. However, smaller companies with heavy dependence on the drug may face revenue challenges.
India has previously invoked Section 26A to prohibit several unsafe drugs and fixed-dose combinations as part of its public health protection measures.
Meanwhile, the government has also intensified efforts to strengthen domestic drug manufacturing. Under the Promotion of Bulk Drug Parks scheme, investments worth ₹4,763.34 crore have been made over the last three and a half years up to September 2025, exceeding the committed investment levels for greenfield projects.
Additionally, the Production Linked Incentive (PLI) scheme for Bulk Drugs, with a total outlay of ₹6,940 crore, aims to reduce India’s dependence on single foreign sources for critical Active Pharmaceutical Ingredients (APIs) and ensure uninterrupted supply of essential medicines.
What's Your Reaction?
 Like
0
Like
0
 Dislike
0
Dislike
0
 Love
0
Love
0
 Funny
0
Funny
0
 Angry
0
Angry
0
 Sad
0
Sad
0
 Wow
0
Wow
0